Enable creativity in complex study design.
Configurable and flexible RTSM technology is key to supporting the evolving needs of your complex clinical trial, from launch through mid-study changes and final close out. With modern technology, you can gain the flexibility needed to ensure your trial runs smoothly, while maintaining robust quality and control.
Navigate unpredictability with ease.
Evolving protocol designs require flexible and robust technology that can support the unknown without impacting patient experience or introducing risk. See how Prancer RTSM® addresses the most pressing issues resulting from complex trials.
-
Protocol Complexity
Learn More
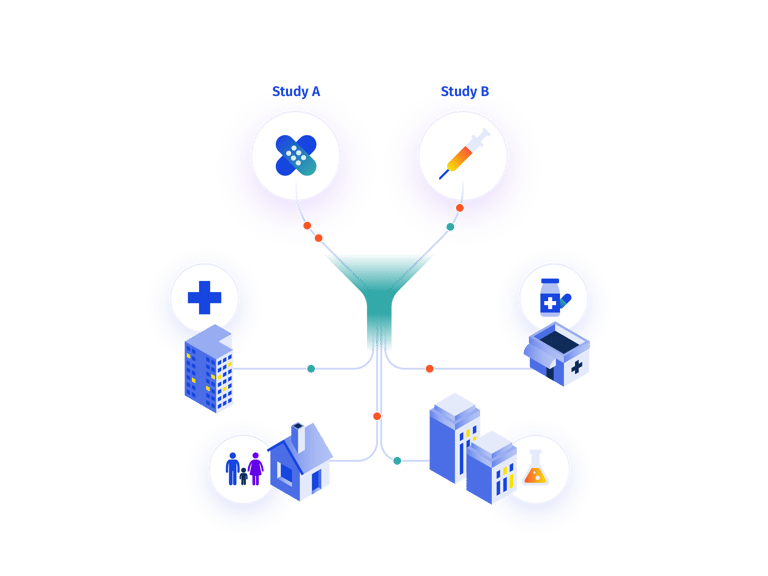
Managing different paths within RTSM for a single study.
Even if an RTSM is designed to handle multiple paths, there are almost always unforeseen changes to the protocol that require amendments. Traditional custom-coded systems treat these amendments as new study builds. Partially-configurable systems still require some level of custom coding, leaving sponsors to choose between working within the limitations of the preset system configurations, or introducing delays while custom code is developed. Prancer RTSM®'s 100% configurability is combined with the ability to add bespoke features, enabling you to be as creative as you need to be in the design and execution of your trials.
-
Supply Complexity
Enable complex supply with infinite configurations.
Tired of choosing between what you want and what your RTSM can do? Let the science lead by adding study-specific logic contained within a configurable solution to control risk and keep timelines short.
-
Operational Burden
Download the White Paper
Alleviate the burden of the RTSM end-user.
Complex clinical trials do not need to be complex for clinical sites. With a robust, modern RTSM like Prancer RTSM® the screens used by sites can be dynamic to ask for the right data at the right time and offer on-screen guidance to help them adhere to the protocol. Study Managers are empowered to adapt the design as the science dictates, controlling the site user experience so they can simply follow the prompts on the screen. By allowing modern technology to automate error prone processes, end-users are able to focus on what matters most, the patient.
Managing different paths within RTSM for a single study.
Even if an RTSM is designed to handle multiple paths, there are almost always unforeseen changes to the protocol that require amendments. Traditional custom-coded systems treat these amendments as new study builds. Partially-configurable systems still require some level of custom coding, leaving sponsors to choose between working within the limitations of the preset system configurations, or introducing delays while custom code is developed. Prancer RTSM®'s 100% configurability is combined with the ability to add bespoke features, enabling you to be as creative as you need to be in the design and execution of your trials.

Enable complex supply with infinite configurations.
Tired of choosing between what you want and what your RTSM can do? Let the science lead by adding study-specific logic contained within a configurable solution to control risk and keep timelines short.

Alleviate the burden of the RTSM end-user.
Complex clinical trials do not need to be complex for clinical sites. With a robust, modern RTSM like Prancer RTSM® the screens used by sites can be dynamic to ask for the right data at the right time and offer on-screen guidance to help them adhere to the protocol. Study Managers are empowered to adapt the design as the science dictates, controlling the site user experience so they can simply follow the prompts on the screen. By allowing modern technology to automate error prone processes, end-users are able to focus on what matters most, the patient.

Case Study
See how a Top 10 Pharmaceutical Company’s leverages Prancer RTSM® to enable intricate, flexible designs within an early phase oncology portfolio

Approve the system, not the specifications.
Traditional RTSM systems require you to sign off on lengthy specifications before building the system. This can lead to unintended issues and surprises during User Acceptance Testing (UAT), causing delays and system rebuilds before study go-live. When you introduce complexity to the study design, those risks only stand to increase.
With Prancer RTSM® you can see the system as early as your first demo, using just the information from your study protocol. Through a sandbox environment, you can test and review the system before ever signing a specification. The results? Increased transparency and alignment and reduced UAT findings and delays.
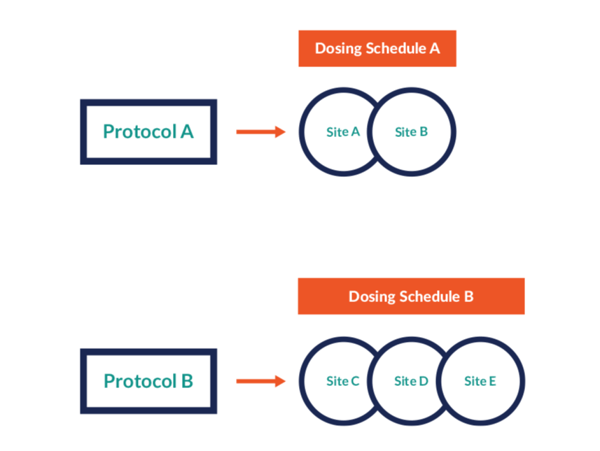
Control protocol versions
Prancer RTSM® enables sponsors to follow positive signals and refine the treatment as data is collected without worrying about individual site approval processes.
- Sponsors can assign the different protocol versions to different sites
- Each version can be connected to a different visit schedule or specific cohort - whatever the protocol change requires
- Per site, any new patient will fall under the new protocol version with whatever that entails
Enable efficiency in complex trials trials with a modern RTSM.
Modern, fully configurable RTSMs offer robust configuration options that support the level of complexity that innovative protocols demand, and reduces the operational and administrative burden.
-
Cohort Management
Flexible cohort management
- Self manage cohorts including opening/closing cohorts, grouping cohorts, defining starting dose levels, and more
- Increase enrollment caps as studies progress to control data collection, discover recommended dose levels or explore expanded targets
- Choose to reserve slots for specific patients or specific sites
-
Dynamic Supply Adjustments
Adaptive supply management
- Lot management: Rely on robust controls built-in to maintain the blind across lots, control access per protocol version, support pooled ancillary supplies, and more
- Expiry control: Support your relabeling efforts through myriad options that follow your real-world work instead of the other way around
- Add countries: Study teams can add countries whenever they are needed and Supply Managers are notified to assign depots and control which lots can be used for those countries
- Cohort-specific resupply: See which cohorts are open and project demand that is specific to the current status of the study
-
Quality
Regression Testing
One of the major headaches that comes with amendments from a systems perspective can be regression testing. With traditional RTSMs, where each amendment is treated as a new study build, there is added complexity, cost, internal resources and added time associated.
Audit Trail
Prancer RTSM® has full transparency into changes with a robust audit trail that is easily queried. For example, the sponsor can see an audit trail of the cohort management, to see who updated a cohort to increase caps or reserve slots per site and when they made each update.
Flexible cohort management
- Self manage cohorts including opening/closing cohorts, grouping cohorts, defining starting dose levels, and more
- Increase enrollment caps as studies progress to control data collection, discover recommended dose levels or explore expanded targets
- Choose to reserve slots for specific patients or specific sites
Adaptive supply management
- Lot management: Rely on robust controls built-in to maintain the blind across lots, control access per protocol version, support pooled ancillary supplies, and more
- Expiry control: Support your relabeling efforts through myriad options that follow your real-world work instead of the other way around
- Add countries: Study teams can add countries whenever they are needed and Supply Managers are notified to assign depots and control which lots can be used for those countries
- Cohort-specific resupply: See which cohorts are open and project demand that is specific to the current status of the study
Regression Testing
One of the major headaches that comes with amendments from a systems perspective can be regression testing. With traditional RTSMs, where each amendment is treated as a new study build, there is added complexity, cost, internal resources and added time associated.
Audit Trail
Prancer RTSM® has full transparency into changes with a robust audit trail that is easily queried. For example, the sponsor can see an audit trail of the cohort management, to see who updated a cohort to increase caps or reserve slots per site and when they made each update.
How Prancer RTSM® Enables Complex Studies
As today’s complex trials evolve, it is essential to choose an RTSM that lets you follow the science.
 The largest part of our portfolio is and always has been complex trials. From basket and umbrella trials to global registrational trials, we've enabled complex protocols and supply chains while collaborating with our clients to let the science lead.
The largest part of our portfolio is and always has been complex trials. From basket and umbrella trials to global registrational trials, we've enabled complex protocols and supply chains while collaborating with our clients to let the science lead.
Kathleen Greenough, Vice President, Marketing & Commercial Operations
